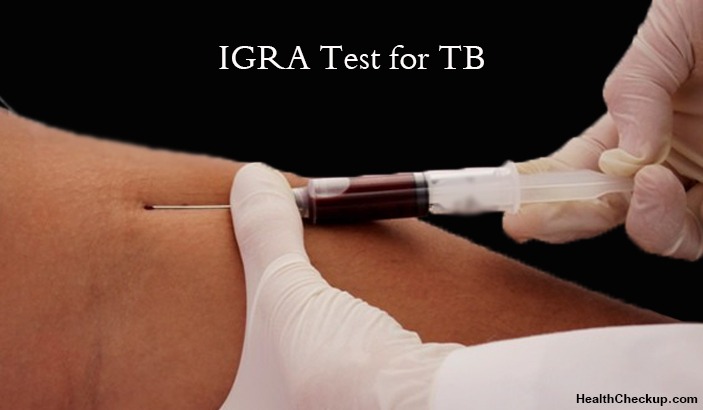
Tuberculosis (TB) is a life-threatening and highly infectious disease that affects the lungs. The bacterium that causes tuberculosis is known as Mycobacterium tuberculosis (Mtb) and it spreads from one person to another through the droplets released into the air through coughs and sneezes. There are strains of tuberculosis that resist the drugs that are used to treat the disease. People suffering from active tuberculosis need to be on medication and treatment for several months in order to eradicate the infection and prevent antibiotic resistance. The interferon gamma release (igra test) assay also known as IGRAs is an important TB blood test that helps in diagnosis of tuberculosis and accessing the effectiveness of the treatment.
What is Interferon gamma release assays (IGRAs) Test?
Interferon-Gamma Release Assays (IGRAs) are in vitro blood tests of cell-mediated immune response that aids in the diagnosis of Mycobacterium tuberculosis infection. IGRA test for TB measures the T cell release of interferon (IFN) -gamma after being stimulated by antigens unique to Mycobacterium tuberculosis bacterium. To conduct these tests, blood samples drawn from the patient are mixed with antigens and controls. This test does not help to differentiate between latent tuberculosis infection and tuberculosis disease.
Types of IGRA Test
Two IGRAS have been approved by the U.s Food and Drug Administration (FDA) are commercially available:
QuantiFERON®-TB Gold In-Tube test (QFT-GIT)
T-SPOT®.TB test (T-Spot)
QuantiFERON®-TB Gold In-Tube:
It is a type of IGRA test that aids in diagnosing Mycobacterium tuberculosis infection in both latent tuberculosis infection and active tuberculosis disease. This test is based on the measurement of a cell-mediated immune response. A combination of three mycobacterial proteins ESAT-6, CFP-10, and TB 7.7 stimulate the patient’s T-cells to release Interferon-gamma, which is measured using ELISA technology. This test is so precise that it can detect infections caused by M. tuberculosis, M. bovis, and M. africanum. Patients who have been vaccinated with BCG or infected with environmental mycobacteria will test negative. The results of this test should be interpreted in combination with other laboratory findings and clinical tests.
Advantages of QuantiFERON®-TB Gold Test
- Better specificity
- Results are not subject to reader bias
- Results are not affected by booster phenomenon
- Results are not affected by BCG vaccination
- Involves only 1 patient visit
- Cost effective test that does not require follow-up testing and treatment
T-SPOT®.TB test:
It is an unique single-visit blood test for tuberculosis screening that works by counting the number of anti-mycobacterial effector T cells, white blood cells that produce interferon-gamma in a blood sample. It gives the total measurement of immune response against mycobacteria which indicate the presence of Mycobacterium tuberculosis bacterium. This test can be used to detect latent tuberculosis because it does not depend on the production of a reliable antibody response or recoverable pathogen. This test used peptides ESAT-6 and CFP-10 which are tuberculosis-specific antigens. This test is comparatively fast and results arrive within 24 hours and it is less affected by previous BCG vaccination.
Advantages of T-SPOT®.TB test
- High sensitivity
- High specificity
- Does not cross react with most non-tuberculosis mycobacteria
- Objective test results
- Involves single patient visit
- Reliable even in challenging populations that are immunosuppressed
Difference between QuantiFERON®-TB Gold In-Tube test (QFT-GIT) and T-SPOT®.TB test (T-Spot):
Initial Process:
- QFT-GIT – Processes whole blood within 16 hours of test
- T-Spot – Processes peripheral blood mononuclear cells within 8 hours, and if -Cell Xtend is used, within 30 hours
M. tuberculosis Antigen:
- QFT-GIT – Single mixture of synthetic peptides containing ESAT-6, CFP-10 and TB7.7.
- T-Spot – Separate mixtures of synthetic peptides ESAT-6 and CFP-10
Measurement Method:
- QFT-GIT – IFN-g concentration
- T-Spot – Number of IFN-g producing cells
Possible Results:
- QFT-GIT – Positive, negative or indeterminate
- T-Spot – Positive, negative, indeterminate or borderline
Disadvantages of IGRAs Test
- Blood samples have to be processed within 8 to 30 hours after collection while the white blood cells in the sample are still viable.
- Errors in collection and transporting the blood samples or in interpreting the assay reduce the accuracy of the IGRAs test.
- Limited data on the use of IGRAs fails to predict if a patient will develop TB disease in future.
- Limited data on the use of IGRAs test on children below the age of 5 years, persons recently exposed to M. tuberculosis bacterium, immunocompromised persons and serial testing.
How to Administer the IGRA Test?
- The IGRA test is done in a qualified laboratory.
- A blood sample is drawn from the patient’s body.
- A follow-up appointment is scheduled for the patient to receive the test results.
- The follow-up evaluation and treatment is decided based on the test results.
IGRA Test Results
- IGRA TB blood test results are based on the amount of IFN-g that is released or the number of cells that release IFN-g. Both the standard qualitative test interpretations (positive, negative or indeterminate) and quantitative assay measurements (nil, TB and Mitogen concentrations) are reported.
- Just like other TB tests, the IGRAs should be used as an aid for diagnosis of M.tuberculosis infection. A positive test result indicates there is an infection; a negative test result indicates that infection is unlikely. Indeterminate result indicates uncertain likelihood of infection and borderline test result also indicates uncertain likelihood of infection.
- The diagnosis of TB disease should combine IGRA test results with other medical evaluation such as chest radiograph, sputum samples and epidemiological and historical information about the patient.
When to Use IGRA Tests?
- IGRAs can be used in place of TST in all situations.
- Persons who have received BCG or who have poor rates of return for TST reading are ideal candidates for IGRAs test.
- TST is preferred over IGRAs for testing children less than 5 years of age.
- IGRAs should not be used for testing persons who have low risk of M.tuberculosis infection.
- Medical institutions and TB control programs should evaluate the availability and benefits of IGRAs in prioritizing their use.
- Routine testing using both TST and IGRA is not recommended.
Interferon gamma release assays blood tests are extremely useful if a patient is at high risk of TB infection but has negative response to skin test. These tests aid the doctor to confirm or rule out latent or active tuberculosis.
Medically Reviewed By